- Inicio
- delta q
- thermodynamics - Confused why delta ∆Q and dQ don't make sense for heat Q - Chemistry Stack Exchange
thermodynamics - Confused why delta ∆Q and dQ don't make sense for heat Q - Chemistry Stack Exchange
4.5 (346) · € 18.50 · En stock
In my chemistry teacher's notes, some notations concerning the heat $Q$ are marked as inappropriate. $Q$: yes d$Q$: no $\delta Q$: yes $\Delta Q$: no In the second bullet in the screenshot below

105 questions with answers in STATISTICAL PHYSICS

The Laws of Thermodynamics
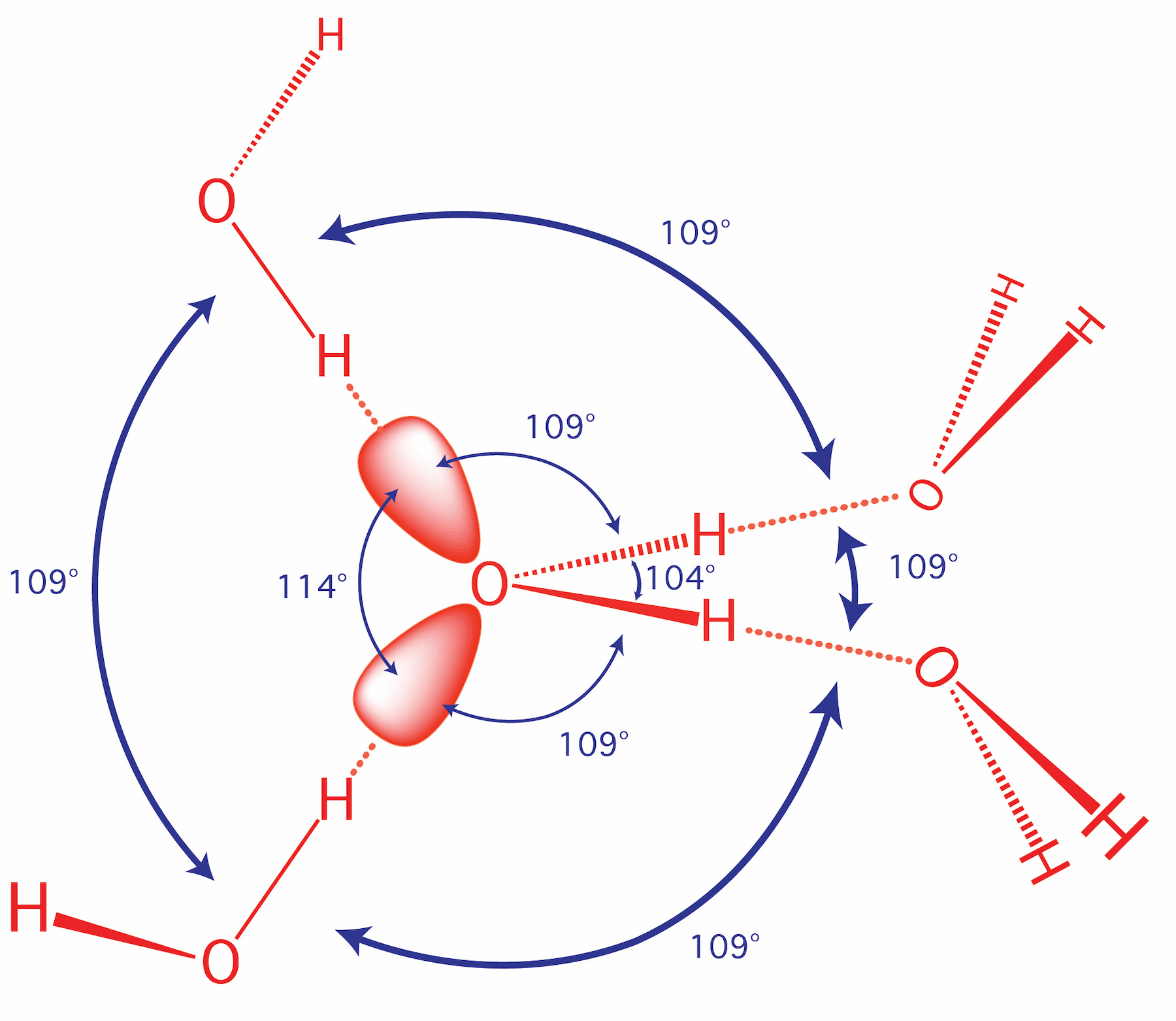
Molecular Interactions (Noncovalent Interactions)

105 questions with answers in STATISTICAL PHYSICS

Thermodynamics, PDF, Thermodynamics

Heat and Mass Transfer - ITI Omar

Computational Physics
In chemistry, how do you use the equation Q=mc∆t? - Quora

Full article: Theory of cross phenomena and their coefficients beyond Onsager theorem
Thermodynamics says, entropy increases with temperature. But can't we say vice versa, increasing entropy increases the temperature? I mean, entropy increases through every act, then doesn't that mean the earth is heating

Quantum Theory of Solvent Effects and Chemical Rea - Survival